Will New FDA Orders Lead to a Recall of All Metal-on-Metal Hip Implants?
May 13, 2011
U.S. Food and Drug Administration orders 21 hip makers to blood test their patients for metal.
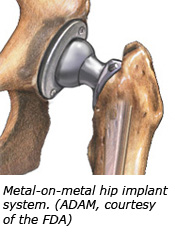 The U.S. Food and Drug Administration, the governmental organization that protects public health, entered the battlefield with the Johnson & Johnson's DePuy recall and ordered 21 manufacturers to collect information from patients - including blood tests for metallic ions. This broad use of the agency's authority will clarify failure rates of metal-on-metal implants and drop the hammer on Johnson & Johnson. The U.S. Food and Drug Administration, the governmental organization that protects public health, entered the battlefield with the Johnson & Johnson's DePuy recall and ordered 21 manufacturers to collect information from patients - including blood tests for metallic ions. This broad use of the agency's authority will clarify failure rates of metal-on-metal implants and drop the hammer on Johnson & Johnson.
"This is a disaster for J&J," said James Moriarty, senior partner at Moriarty Leyendecker. "It will be a public health nightmare and show how metal-on-metal hip implants can cause metallosis." (the swelling around metal implants as a result of corrosion or an allergic reaction).
What could this mean for Johnson & Johnson and the healthcare industry?
- New data could magnify the defects of the recalled DePuy ASR.
- Will create an apples-to-apples study for the DePuy ASR to be compared to other devices.
- Will cause pandemonium in hospitals as all metal-on-metal hip patients rush to test for metal in their blood.
- Send lawyers, lawsuits and patients swarming after irresponsible hip manufacturers.
- Cause the recall of the DePuy's Pinnacle Acetabular device, another device that is repeatedly failing with hip patients.
In 2010 doctors implanted a nurse with the DePuy ASR Pinnacle device. After the procedure, the patient complained, "The pain in groin is worst when I lift left leg 45, 60 and 90 degree, it feels like a click/catch and the pain is worst at those points. I am a registered nurse and have taken care of pts [patients] with hip replacements, this is not normal recovery. Something is wrong with this device." These complaints are typical of patients suffering from metallosis after hip implant surgery.
The risk of metal-on-metal devices is that metal may enter a patient's bloodstreams after the procedure as tiny particles wear off the device and enter the space around the implant. The FDA stated its concerns in a February 2011 report, "Concerns about Metal-on-Metal Hip Implant Systems" The report spotlights the uncertainty of the device's failure rates - hence the need for more studies.
New information must be submitted to the FDA within 30 days and could lead to a recall of all metal-on-metal hip replacement devices. It's the first battle in the war on metal-on-metal hip makers that could destroy Johnson & Johnson and DePuy. |
